This aims to excludes high-carbohydrate foods including such as some fruits and vegetables, bread, pasta, grains and sugar, while increasing the consumption of foods high in fat such as nuts, cream, and butter. A similar diet, called the Atkins diet, restricts carbohydrates to approximately 40–60 g per day and the types of carbohydrates consumed are restricted to those that have a glycaemic index lower than 50.
Biochemical mechanisms
Fats as energy sources: Fats (lipids) are stored in adipose tissue. These stored fat molecules are synthesized in the body from the glycerol and fatty acids in a process known as lipogenesis. When the blood glucose drops the levels of insulin also drops and the levels of glycagon increases. This stimulates the release of triglycerides and Free Fatty Acids (FFA) from the adipose stores and mobilized in to the blood. As these flow, via the blood stream, through the liver the triglycerides are broken down to glycerol and fatty acids. As mentioned above glycerol is then converted into one of the intermediate products of glycolysis to produce pyruvic acid which then enters the Krebs cycle. Fatty acids are changed via a series of reactions called beta-oxidation into acetyl CoA molecules, which also enter the Kreb’s Cycle. The enzyme carnitine, is important in this process as it is also increased by glycagon and enhances the utilization of FFA by increasing their transport into the mitochondria of liver cells where the conversion to acetyl CoA occurs (see picture below). Cells can happily transfer from sugar to fatty acid use throughout the day depending on the proximity to meals and amount of sugar in the blood stream. Obviously, if an individual never lets their blood glucose levels drop by snacking constantly, fatty acids are used less, fat stores are not ultilised and obesity sets in. The corollary of this is after fasting in which fatty acid conversion to acetyl CoA (in the liver) is enhanced and a third water soluble energy source call ketones is formed which is particularly useful for the brain which cannot directly use fatty acids for energy. This is highlighted in more detail below:
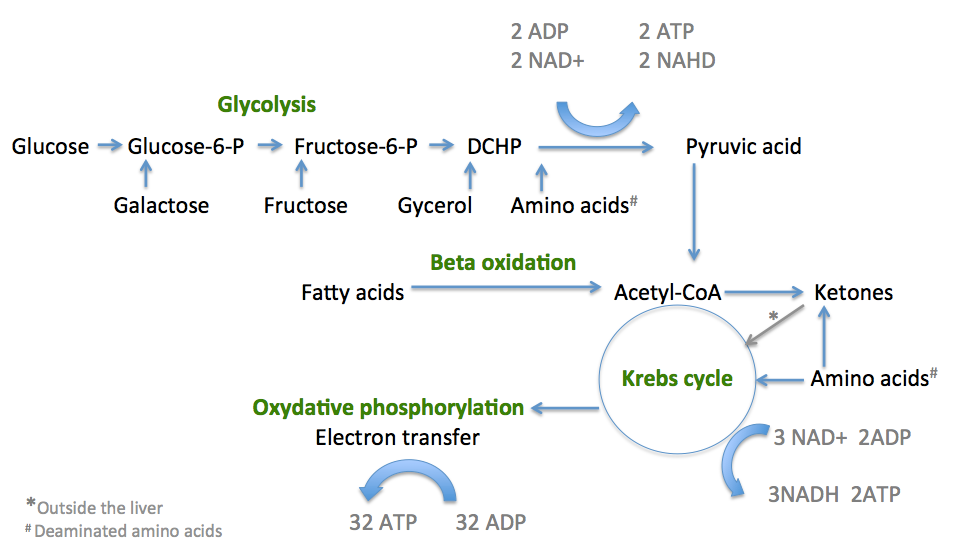
Summary of the three sections of cellular respiration
Proteins as energy sources: Proteins can also be used cellular energy sources. When you eat proteins in food, your body has to break them down into amino acids before they can be used by your cells. Most of the time, amino acids are recycled and used to make new proteins, rather than oxidized for fuel. However, if there are more amino acids than the body needs, or if cells are starving, some amino acids will broken down for energy via cellular respiration. In order to enter cellular respiration, amino acids must first have their amino group removed in a process called deamination. This step makes ammonia as a toxic waste product, and in humans enzymes, in the liver, then convert it to urea and uric acid by adding carbon dioxide which is the then filtered and excreted by the kidneys.
Once the amino acids have been deaminated, products called alpha-keto acids are formed which enter the cellular respiration pathways at different stages depending on the type of amino acid they originated from (there are 20) and what type of deaminated product is formed. Some are ketogenic amino acids which can be degraded directly into acetyl CoA and ketone bodies which are used in the Krebs cycle where they are ultimately degraded to carbon dioxide. This is in contrast to the glucogenic amino which can enter the glycolysis pathway to form pyruvic acid.
Some intact amino acids are import for the maintenance of the Krebs cycle so are important for energy production. In particular, the non-essential amino acid glutamine has been generating academic interest recently. Glutamine serves as an inter-organ shuttle of carbon and nitrogen. It therefore helps cells import nitrogen which need it and clear it from cells which do not. It is a major source of nitrogen for other non-essential amino acids and nuclear (nucleotides). Glutamine is also needed to produce both acetyl-CoA and oxaloacetate (OAA) which condensate produce citrate in the first part of the Krebs cycle activity. In starvation, less puruvate enters the cycle via glycolysis but the cell is able to upregulate the glutamine metabolism to generate both oxaloacetate and acetyl-CoA, enhancing krebs cycle function. Early laboratory work removing glutamine from cancer cell nutrients has resulted in reduced growth but this would be impractical in humans as glutamine is ubiquitous in meat, fish, pulses and vegetables.
Ketones as energy sources
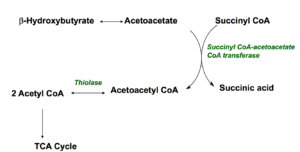
As you have just read, ketones are produced when the body burns fats or proteins to produce energy molecules. They are also produced when there is not enough insulin to help your body use sugar for energy such as uncontrolled type 1-diabetes. Fatty acids are changed via a series of reactions called beta-oxidation into acetyl CoA molecules which enters the Kreb cycle to produce energy molecules. If fatty acids are used primarily acetyl CoA also produces ketone bodies. Three most reported are; acetone which is volatile and excreted in the breath; B-hydroxybutyrate which is converted to acetoacetate and acetoacetate itself. To understand why this mechanism an survival advantage, it is important to note that liver cells lack the enzyme (Succinyl CoA transferase) which allows ketones to utilize ketones as energy so instead ketones are released into the blood stream in order to feed other tissues such as the brain, muscles and heart. In these tissues they are converted back to acetyl CoA which then enters the TCA (Krebs) cycle to produce energy:
The ketogenic diet also favours medium chain triglycerides (MCT) such as coconut oil which have fatty acids which more easily converted to acetyl CoA to be used for energy in the Krebs cycle or converted to ketones in the liver – i.e. they are more ketogenic. This also means that as less overall MCT fats are needed to trigger a ketogenic effect, a greater proportion of carbohydrate and protein can be consumed, allowing a greater variety of food choices. MCT are also more efficiently transported to the liver via the hepatic portal vein rather than, more slowly, via the lymphatics.
A ketogenic diet will lower blood sugar within 3 days and the brain will then get 30% of its energy from ketones. After 40 days 70% this goes up to 70% but the brain will always retain some need for glucose. The ketogenic effect or ketosis is not the same as ketoacidosis, which occurs with very high sugars and insulin deficiency in type-one diabetes or sever starvation. Nevertheless, regular users of the ketogenic diet monitor their urine for ketone bodies to ensure they are not going to far and various commercial monitors are available which seem very sensible.
The biochemical changes which occur in cancer cells associated with the ketogenic diet were considered by Warburg to be caused by an irreversibly impaired in its mitochondrial function forcing cells to use aerobic glycolysis instead. This view is challenged by recent investigations, which found that the function of mitochondrial OXPHOS in many cancers is intact and enhanced glycolysis suppresses OXPHOS rather than defects in mitochondrial. There are a number of other factors, which dispute the so-called Warburg effect. They are also exhibit greater plasticity than normal tissues able to change their metabolic preference to adapt to micro-environmental changes. If glycolysis is inhibited in cancer cells, for example by reducing glucose levels through starvation, the function of mitochondrial OXPHOS is simply restored and fatty acids and ketones can be metabolized. In cancer cells, since the glycolytic contribution to total ATP production does not generally exceed 60%, the rest of the ATP production still comes from the Krebs cycle cycle and OXPHOS. In the presence of hypoxia, however, the contribution of energy production from OXPHOS is reduced to below 30% so there is an even greater dependence on glycolysis. This also confirms that the preference of cancer cells to use glycolysis is primarily triggered by hypoxia rather than a defect in mitochondria especially as they have also learnt to suck – up glucose a high rate for aerobic glycolysis even when serum levels are low.
It has also been widely quoted that cancer cells prefer glycolysis because, relative to normal cells, they demonstrate significant alterations in metabolism that result in increased levels of damaging reactive oxygen species (ROS) which could damage and potential kill them. It has also been proposed that cancer cells increase glycolysis and shift from OXPOS because the later forms more ROS as a side product. Given this theoretical construct, it is reasonable to propose that forcing cancer cells to use mitochondrial oxidative metabolism by feeding ketogenic diets that are high in fats and low in glucose and other carbohydrates, would selectively cause metabolic oxidative stress in cancer versus normal cells. Increased metabolic oxidative stress in cancer cells would in turn be predicted to selectively sensitize cancer cells to conventional radiation and chemotherapies. This hypothesis is also probably not true as recent research has shown that some cancer cells have learnt to develop resistance to oxidative damage by decode oxidative-stress signals and convert them into pro-survival signals actually promoting growth even in highly oxidative environments.
It appears therefore that the general held underlying hypothesis for the ketogenic diet is fundamentally flawed. That does not mean that a diet of reduced sugars and carbohydrates and higher fats does not have significant health benefits. The is well known to help children with epilepsy, improves diabetic control lowering hemoglobin A1C levels; helps with weight loss and there may well be direct and anti-cancer benefits. Certainly, The Warburg effect was support by early laboratory experiments have found that proliferation and tumorigenicity of cancer cells was marginally reduced when glycolysis activity was suppressed, suggesting that enhanced OXPHOS is still not sufficient to meet all the requirement of cancer growth. In animal and some limited human studies the ketogenic diet demonstrate tumour slowing effects. The main reason for these benefits however lays in its avoidance of processed sugars and carbohydrate the multitude of benefits and underlying direct and indirect biochemical pathways are addressed in our section so this website.
The ketogenic diet also has a number of other pitfalls.
- It excludes some fruits and vegetables which are rich sources of vitamins, minerals, polyphenols and fibre.
- It can cause weight loss, a problem for some vulnerable patients
- It favours animal proteins and does not exclude carcinogenic cooking methods.
- It can cause constipation, raised cholesterol, kidney stones and shunt growth in children.
.
What is the optimal diet for long term health:
 Hopefully, with all the information on this website, you will be convinced of a more comprehensive, sustainable dietary plan which involving:
Hopefully, with all the information on this website, you will be convinced of a more comprehensive, sustainable dietary plan which involving:
- Low glycaemic index foods .. more
- High in plant fats, vitamins, essential minerals ..more
- High in healthy plant fats ..more
- Low in unhealthy saturated animal fats ..more
- High in fibre ..more
- High in plant proteins ..more
- Low in carcinogenic meats ..more
- High in probiotic bacteria ..more
- High in polyphenols and other healthy phytochemicals ..more