Diet and Arthritis | Exercise and arthritis | About arthritis | References (arthritis)
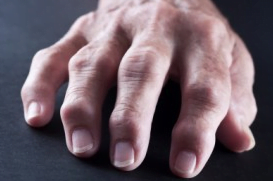 Studies have demonstrated a strong link between diet and arthritis. People who eat foods particularly rich in polyphenols (Fruit, vegetables, teas, herbs, spices, pulses), have been shown to have a lower risk of osteoarthritis-related musculoskeletal inflammation [Wang, McAlindon, Williams, Hanninen]. Similarly, another study confirmed that a period of fasting, followed by a diet rich in cruciferous vegetables, helped lower the number of tender swollen joints, decrease the duration of morning stiffness, improve grip strength and reduced markers of chronic inflammation [Kjeldsen-Kragh].
Studies have demonstrated a strong link between diet and arthritis. People who eat foods particularly rich in polyphenols (Fruit, vegetables, teas, herbs, spices, pulses), have been shown to have a lower risk of osteoarthritis-related musculoskeletal inflammation [Wang, McAlindon, Williams, Hanninen]. Similarly, another study confirmed that a period of fasting, followed by a diet rich in cruciferous vegetables, helped lower the number of tender swollen joints, decrease the duration of morning stiffness, improve grip strength and reduced markers of chronic inflammation [Kjeldsen-Kragh].
Dietary features which increase inflammation:
- Processed sugar and refined carbohydrates – tips to reduce sugar intake
- Excess alcohol consumption – tips for sensible alcohol intake
- Food allergies to substances such as gluten and lactose which cause chronic gut inflammation
- Fried and processed foods
- Advanced glycation end products (AGE), toxins that appears when foods are grilled, fried, or pasteurised
Dietary measures which reduce chronic inflammation:
- Eating more plant-based proteins (e.g. Soy, lentils, quinoa, chickpeas, beans, buckwheat)
- Eating more fruit and vegetables
- Eating more polyphenol-rich foods – see below
Particular benefits of polyphenol-rich foods
Unlike conventional drugs which simply relieve the symptoms of arthritis, polyphenol-rich foods are capable of preventing the onset and progression of underlying joint damage via a number of biochemical pathways:
- Anti-inflammatory properties, which reduce discomfort and stiffness
- Antioxidant properties, which protect the joint from oxidative damage [Giovannucci, Hanninen]
- Direct anti-apoptotic effects on chondrocytes, reducing cartilage degeneration [Shen].
- Modulation of metalloproteinases which can thin cartilage [Dahlberg, Mitchel, Brinckerhoff].
A wide variety of polyphenols and other phytochemicals have been subject to cell line, animal and human experiments in order to assess their effectiveness at improving joint health, and much of the evidence for their effectiveness has been summarised in an Arthritis Research review [Aeuk]. In general, plant foods which are colourful, tasty and have a nice aroma are rich in polyphenols, with rosehip, turmeric (curcumin), green tea, boswellia serrate, cordyceps militaris, pomegranate, and broccoli, all shown to be particularly effective. Here are some recipes which embrace the anti-inflammatory high polyphenol, high plant protein philosophy:
Specific foods and food supplements

Glucosamine, an amino sugar made either from shellfish or prepared in a laboratory setting, has been found in animal studies to delay the breakdown and improve repair of damaged cartilage [Fox, Towheed]. In humans with established arthritis, the results from 13 RCTs are mixed [Towheed]. While one RCT involving 400 women with breast cancer did demonstrate a significant difference in pain and knee joint space [Bruyere], two large meta-analyses concluded no meaningful benefit from and glucosamine suggested that further research was needed [Wandel, Towheed].
Chondroitin is a complex sugar found in cartilage usually made commercially from cows, pigs and sharks. Laboratory studies have found it can reduce the activity of enzymes that break down collagen in joints while it is known to have anti-inflammatory properties [Thomas K]. In humans, some RCTs have demonstrated a benefit over placebo, but two meta-analyses concluded that no clinically meaningful benefits could be attributed to regular supplementation [Reichenbach, Wandel].
Fish oils have been reported to improve symptoms of rheumatoid arthritis and also enables lower intake of non-steroidal anti-inflammatory drugs, in turn, lowering the risks of cardiovascular, renal and gastric disease. Evidence suggesting that consumption of fish liver oil improves osteoarthritis currently lacks sufficient data [Cleland 2006, Fortin 1995]. A recent RCT involving women taking aromatase inhibitors found no that fish oil had no benefit over placebo [Hershman 2015].
Rosehip (Rosa canina), a species of wild rose native to Europe, Africa and Asia, contains vitamin C and is rich in anthocyanins, flavonoids (quercetin and catechin) and galactolipids [Thomas]. Rosehip has demonstrated anti-inflammatory capabilities in several experimental models [Deliorman, Winther]. The anti-inflammatory mechanism of the polyphenols within rosehip has been explained via its metabolism of arachidonic acid and inhibition of both cyclooxygenase-1 and 2 [Lattanzio]. Rosehip has also been found to confer chondroprotective effects in vitro [Schwager]. Its clinical effect on joint health has been highlighted in two systemic reviews [Rossnage lK, Chrubasik C]. These included 4 clinical studies, which, although small, suggest a meaningful benefit in humans. Intake of 4 g a day over a four-month period reduced both joint swelling and painkiller use when compared to a placebo.
Turmeric (Curcuma longa) is a perennial plant native to southern Asia and originating from the ginger family. Its constituents include the three curcuminoids, demethoxycurcumin, bisdemethoxycurcumin and curcumin (diferuloylmethane), the primary constituent and the one responsible for its vibrant yellow colour, as well as volatile oils (turmerone, atlantone and zingiberene)[Thomas]. Pharmacological activities, including antioxidant and antimicrobial properties, have been attributed to these curcuminoids [Handler, Thomas]. Cell culture and animal research trials indicate that curcuminoids may have a role in treating diseases such as inflammatory bowel disease, pancreatitis and chronic anterior uveitis [Jurenka 2009]. In addition to these mechanisms, turmeric has antioxidant properties [White].
In one animal experiment, turmeric was administered on a 1:1 random basis intraperitoneally to rats prior to the onset of streptococcal cell wall-induced arthritis. The turmeric profoundly inhibited joint inflammation and periarticular joint destruction in a dose-dependent manner. It also prevented local activation of NF-kappaB and the subsequent expression of NF-kappaB-regulated genes which mediate joint inflammation and destruction via including chemokines, cyclooxygenase 2 and RANK. Consistent with these findings, inflammatory cell influx, levels of prostaglandin E(2) in the joints, and periarticular osteoclast formation were inhibited by the turmeric extract treatment [Funk].
In humans, a crossover double-blind RCT involved either a placebo or 50mg of turmeric in combination with zinc complex (50 mg/capsule), as well as other botanicals such as Boswellia serrate, being given to 42 patients with osteoarthritis three times daily for three months. Those taking the turmeric combination demonstrated significant improvements in pain severity and disability scores [Kulkarni]. Another study, involving 100 men and women with a history of joint pain, involved participants being randomised to receive either a placebo or a supplement containing turmeric root extract. Participants given the turmeric extract reported an improvement in joint pain severity, improvements in the ability to perform daily activities and improved stiffness scores and knee pain after 8 weeks [Nieman]. Finally, a study involving 107 participants with arthritic pains of the knee randomly compared the effects of taking 2g of turmeric versus 800mg ibuprofen per day for 6 weeks. Although both groups’ pain levels improved when walking and climbing stairs, improvement was significantly greater among the turmeric group [Kuptniratsaikul]. Regarding safety, these and other studies have reported turmeric having minimal adverse events, even with intake over 3g/day [Shah, Sharma, Loa].
Green tea (Camellia sinensis) is a rich source of EGCG (Epigallocatechin 3-gallatethe) [Thomas, Manning, Cooper R], a potent antioxidant activities 25-times more effective than vitamins C [Doss, Cooper]. Several laboratory studies analysing chondrocytes derived from OA cartilage have demonstrated that pre-incubation with tea extract, or pure EGCG, reduced pro-inflammatory cytokines such as IL-1β, TNFα, IL-6, prostaglandin E2 and COX-2 [Ahmed, Singh, Samuels, Malemud]. Tea catechins, via their influence on inflammatory cytokines, block activation of matrix-degrading enzymes known as matrix metalloproteinases (MMPs) [Ahmed-5, Brinckerhoff CE, Adcocks, Vankemmelbeke], a large group of collagenase enzymes expressed in high levels in arthritic joints which promote cartilage degradation [Brinckerhoff, Goo].
In animals, the potential impact of EGCG on improving arthritis was first discovered in a study in which mice with collagen-induced arthritis (CIA) given drinking water containing EGCG experienced clear improvements [Haqqi]. Both CIA incidence and severity were reduced, with the mice given ECGC experiencing a marked inhibition of the inflammatory mediators COX-2, IFNγ, and TNFα. Additionally, total immunoglobulins (IgG) and type II collagen-specific IgG levels, were found to be lower in the arthritic joints of mice exposed to EGCG [Haqqi]. Despite this cell line and animal data, there are no significant, prospective trials for humans with arthritis in the pipeline, although studies investigating other conditions in humans have confirmed EGCG’s excellent safety profile [Taylor, Heck, Salahuddin, Cooper].
Pomegranate (Punica granatum L) is a tree fruit grown in hot dry countries. In the past decade, numerous studies on the antioxidant, anti-carcinogenic and anti-inflammatory properties of pomegranate constituents have been published, focusing on the treatment and prevention of; cancer, cardiovascular disease, diabetes, dental conditions, male infertility, Alzheimer’s disease, arthritis, and obesity [Jurenka]. It contains a variety of phytochemicals including the antioxidant punicalagin, and the polyphenol gallic acid. One study has demonstrated that pomegranate possesses significantly greater antioxidant capacity at much lower concentrations (>1000-fold dilutions) than either grape or blueberry juice, a finding attributed to the higher anthocyanin flavonoid and total flavonoid content than the other juices [Ignarro]. This antioxidant capacity is further enhanced by using the whole fruit, as opposed to extracting individual chemical constituents [Lansky]. In animal and in vitro studies, whole fruit pomegranate extract has been shown to inhibit both the lipoxygenase and cyclooxygenase enzymes which are key to the conversion of inflammatory mediator arachidonic acid to prostaglandins [Schubert].
Pomegranate has also been shown to have a significant inhibitory effect on MMPs within arthritic joints, primarily by inhibiting IL-1beta- induced destruction of proteoglycan [Ahmed]. In humans, no randomised trials specifically investigating joint health have been performed, but one small pilot study demonstrated that pomegranate extract reduced joint tenderness, while participants also had lower serum markers of oxidative stress [Balbir-Gurman].
Broccoli is a cruciferous vegetable rich in phytochemicals such as isothiocyanate and its metabolite sulforaphane (SFN). These have been found to have significant health benefits and improve several conditions including cancer, atherosclerosis and a variety of inflammatory disorders [Thomas, Juge, Dinkova-Kostova, Zakkar]. Brocolli phytochemicals are thought to exert their influence via a variety of biochemical mechanisms. They are known to be potent inductors of antioxidant enzymes, particularly glutathione, which bind free radicals formed by dietary or environmental carcinogens before they damage our DNA, including those within the tissues of our joints [Moore LE et al 2007, Cornelis MC 2007). SFN has been shown to modulate MMP expression in chondrocyte cell lines [Megias J, Kim HA, Kong JS, Young D]. In animal models of arthritis, mice fed an SFN-rich diet had significantly reduced cartilage destruction after 12 weeks, compared with those fed a control diet. This is thought to be due to SFN’s effectiveness at reducing the activation and production of interleukin-17 and tumour necrosis factor [Chen WP, Culley, Kong JS]. Another recent animal study, led by the School of Biological Sciences and Norwich Medical School, found that sulforaphane blocked histone deacetylase (HDAC) inhibitor as well as MMP expression, resulting in direct chondroprotective effects [Davison].
Cordyceps militaris, a parasitic caterpillar fungus found in humid tropical countries, is rich in the phytochemical cordycepin, also known as 3-deoxyadenosine, a nucleoside derivative [Won]. Various studies have focused on the pharmacological activities of cordycepin and revealed it has anti-inflammatory, anti-angiogenesis, anti-ageing and anti-proliferation properties [Kim, Yoo, Lee, Lee, rao]. One recent study examining the inhibitory effects of cordycepin on IL-1β induced osteoarthritis (OA) revealed that cordycepin protected cartilage via its ability to decrease glycosaminoglycan (GAG) release. This study also highlighted several other properties of cordycepin which help prevent OA, namely inhibition of both the gene expressions of MMP catabolic enzymes and inflammatory mediators such as Cyclo-oxidase-2 [Penfei]. Studies involving human cartilage and chondrocytes have confirmed cordycepin decreases GAG release, lowers nitric oxide production, and decreases gene expressions of inflammatory and catabolic mediators [Rao]. Extracts have been found to be completely safe in toxicology studies [Yan, Yooh].
Boswellia serrate (Indian Frankincense) is a branching tree of the family Burseraceae that grows in dry mountainous regions of India, Northern Africa and the Middle East. Its resin contains a number of phytochemicals including; monoterpenes, diterpenes, triterpenes, pentacyclic triterpenic acids (boswellic acids), and tetracyclic triterpenic acids [Siddiqui]. These phytochemicals, particularly the boswellic acids, have a wide range of microbiological effects which have potential disease-modifying effects on arthritis and other chronic conditions [Siddiqui].
In human chondrocytes, Boswellia serrata has demonstrated an ability to suppress a protein known as RANKL, which induces osteoclastogenesis and potentiates apoptosis, preventing cartilage and bone reabsorption [Sengupta, Tardaka]. Boswellia has also been shown to suppress the TNF-α induced release of MMP-3 [Siddiqui, Sengupta]. A unique property of boswellic acid is its ability to inhibit leukotriene synthesis by either interacting directly with, or blocking, translocation of the pro-inflammatory enzyme 5-lipoxygenase (5-LO) [Siemoneit]. Boswellia serrata also inhibits NF-kB activation from irritants such as cigarette smoke or direct exposure to cytokines [Siddiqui].
In mice with formaldehyde induced arthritis, those fed with Boswellia exhibited a 50% increase in anti-arthritic activity [Singh]. A similar protective effect was seen in rabbits who had arthritis induced by bovine serum albumin [Sharma]. In humans, an RCT involving 75 participants with established osteoarthritis found that, compared to placebo, those given enriched Boswellia serrata extract had improved pain and functional ability scores as well as a significant reduction in synovial fluid matrix metalloproteinase-3 [Sengupta]. In another small RCT, capsules containing 333 mg of Boswellia taken three times a day for 8 weeks were found to improve pain, knee flexion and walking distance compared to placebo [Kimmatkar]. Several small, non-controlled randomised trials involving participants with rheumatoid arthritis report similar benefits [Kulkarni, Chrubasik, Chopra].
Beetroot (Beta vulgaris rubra), while rich in several bioactive compounds including ascorbic acid, carotenoids, phenolic acids and flavonoids, is also one of the few vegetables that contain a group of phytochemical pigments known as betalains. These are categorised as either betacyanin pigments which are red-violet in colour, or betaxanthin pigments which are yellow-orange [Clifford, Ninfali]. A number of investigations have reported that betalains have high antioxidant and anti-inflammatory capabilities [Vidal, Clifford, Reddy], suggesting that beetroot may have a role in the nutritional management of clinical pathologies characterised by oxidative stress and chronic inflammation, including arthritis [Pietrzkowski] and even cancer [Das].
Betalains have been shown to interfere with pro-inflammatory signalling cascades involving Nuclear Factor-Kappa B (NF-κB) and interleukin-6 (IL-6) [Baker], while they also appear to markedly suppress the expression of cyclooxygenase-2 (COX-2), a precursor molecule for pro-inflammatory arachidonic acid metabolites and prostaglandins [Vidal]. One study group reported that betalains inhibited COX-2 enzyme activity by 97%, a level comparable to or greater than commonly used anti-inflammatory drugs (Ibuprofen, and Celebrex). In humans, therapeutic administration of betalain-rich oral capsules made from beetroot extract alleviated inflammation and pain in osteoarthritic patients. Additionally, after 10 days of supplementation (100, 70 or 35 mg per day), the pro-inflammatory cytokines, tumour necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), had decreased by up to 30% [Pietrzkowski].
The antioxidant properties of betalain, and other pigments in beetroot, have been reported in several animal studies [Reddy, Clifford]. In one such study, rats were randomised to receive either a normal diet or a diet supplemented with a dried beetroot extract for 7 days, before being exposed to carbon-tetrachloride, a well-established carcinogen and reactive oxygen, nitrogen species (RONS) generator. Those rats pre-treated with the beetroot expressed significantly lower levels of lipid peroxidation, a marker of oxidative damage. Furthermore, the beetroot extract-fed rats maintained endogenous antioxidant enzyme activity (glutathione peroxidase, superoxide dismutase and catalase enzymes) at normal cellular concentrations following the oxidative insult, while those in the control group saw levels drop significantly. This suggests beetroot exhibits indirect antioxidant effects via up-regulation of antioxidant defence mechanisms [Vulić].
Piperine (Piper nigrum and Piper longum, respectively) is a constituent of black pepper and long pepper. Studies have shown it increases the bioavailability of polyphenols following ingestion of other foods such as curcumin, a chemical which is not particularly well absorbed on its own [Belcaro]. In humans, 20mg piperine given concomitantly with 2g of curcumin increased serum curcumin bioavailability 20-fold [Shoba]. In another study, complexing curcumin with a phospholipid increased absorption 5-fold [Belcaro]. It has also been shown to increase to absorption of polyphenols from other foods [Belcaro].



